
Research Summary
Conjugation in the streptomycetes. Members of the mycelial spore-forming bacterial genus Streptomyces are renowned for their ability to synthesize antibiotics. They are also remarkable for a novel process of conjugation that appears to involve the transfer of double-stranded DNA. As the details of this process remain largely obscure, our aim is to elucidate the exact mechanism by which DNA, both chromosomal and plasmids, is transferred between Streptomyces mycelia. The circular plasmid pIJ101 (Fig. 1) encodes two functions required for efficient intermycelial transfer of the plasmid- the tra gene whose protein product also mediates chromosome transfer and a cis-acting locus of transfer (clt).

Fig. 1. Genetic and physical map of the conjugative Streptomyces
plasmid pIJ101.
The minimal clt locus is comprised of 54-bp, includes both indirect repeat (IR) and direct repeat (DR) sequences, and is present within a region of pIJ101 that shows intrinsic curvature (Fig. 2). Three additional pIJ101 genes (spdA, spdB, and kilB) participate in the poorly understood process termed plasmid spread, which may involve movement of plasmid copies throughout recipient mycelia.
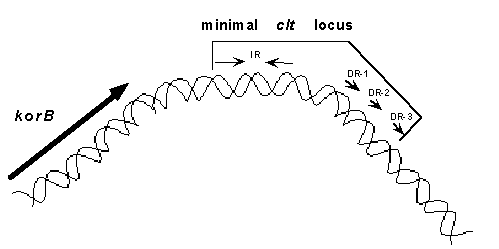
Fig. 2. Computer modeling of intrinsic curvature within
the region of plasmid pIJ101 that includes the 54-bp minimal clt
locus.
Pathogenicity in the streptomycetes. We are also interested in plant pathogenic Streptomyces; in particular, the sweet potato pathogen Streptomyces ipomoeae, which causes the necrotic disease known as soil rot. We have purified an anti-microbial substance (ipomicin) that kills a subset of S. ipomoeae strains in a highly specific manner (Fig. 3). We aim to isolate a separate inhibitory substance that is known to inhibit or kill the remaining subset of S. ipomoeae strains in our collection. Together, these compounds may represent a separate method for controlling this disease. We have also begun to isolate and characterize the S. ipomoeae genes required for pathogenicity. Some of these genes are expected to be unique to S. ipomoeae because of certain distinct biochemical and disease etiology features of this organism. All pathogenicity determinants in S. ipomoeae may be part of a pathogenicity island, which has already been identified in other Streptomyces pathogens and which has been shown to be transmissible in one of them.
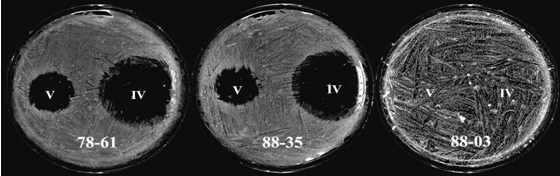
Fig. 3. Plate bioassays in which purified ipomicin protein
(designated IV and V here) is spotted onto agar plates spread with spores
of the susceptible S. ipomoeae strains 78-61 or 88-35 or the
resistant S. ipomoeae strain 88-03. Reactions shown are as seen
following a three-day incubation of the plates at 30°C.
Phase variation and biofilm formation in Vibrio vulnificus. The pathogenic marine bacterium Vibrio vulnificus accounts for the vast majority of deaths associated with the consumption of raw shellfish such as oysters. Following ingestion, a rapidly fulminating septicemia can ensue, with death occurring in as little as 24 hours. Those most susceptible include individuals who are compromised for either liver or immune system function, or who are diabetic. Virulent V. vulnificus secrete a polysaccharide capsule which encases each cell, making colonies of these cells appear opaque. In contrast, colonies of unencapsulated cells appear translucent and are avirulent. Switching between opaque and translucent cell types occurs by an uncharacterized mechanism referred to as phase variation. We are interested in defining the mechanism(s) of phase variation in V. vulnificus, including any environmental signals that may regulate the process. By understanding the mechanism and regulation of phase variation, it may be possible to control the process and thereby prevent the maintenance and/or formation of encapsulated virulent strains in oysters.
We recently discovered and characterized an additional potentially virulent phenotype of V. vulnificus known as rugose (Fig. 4), where cells are encased in polysaccharide that is distinct from the capsule surrounding opaque cells. Both opaque and translucent strains can yield rugose variants, and vice versa; however, it is unknown if a single mechanism governs both the switch between opacity and translucence and the switch between these forms and the rugose phenotype. Most significantly, we found that biofilm production for rugose variants is about 20 to 40 fold greater than that of the other variants. Biofilms are bacterial populations enclosed in an extracellular matrix that adhere to surfaces and promote survival under adverse environmental conditions. It is possible that the biofilm-proficient rugose form of V. vulnificus allows persistence of the organism in marine environments, perhaps even within oysters. Similar survival arguments during periods between cholera epidemics have been made for the Vibrio cholerae rugose form. As environmental control of biofilm formation has also been demonstrated for Vibrio species, an assessment of the environmental parameters most essential for production of biofilms by V. vulnificus may elucidate important controlling factors. From these studies, new strategies for reducing or eliminating the presence of V. vulnificus in oysters may be developed, which focus on preventing formation or maintenance of biofilms.
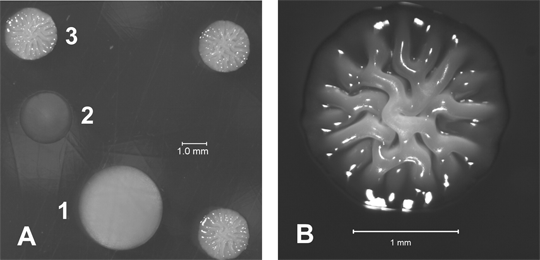
Fig. 4. V. vulnificus phase
variants. (A) 1, opaque; 2, translucent; 3, rugose. (B) Rugose colony
at higher magnification.